|
| |
TRUCK SERVICE MANUAL
TM 5-4210-230-14&P-1
a specific gravity of 1.835. Water has arbitrarily been
assigned a value of 1.000. Therefore, electrolyte With a
specific gravity of 1.265 means it is 1.265 times heavier than
pure water.
The state-of-charge of a battery can be determined by the
specific gravity of the electrolyte. The specific gravity can be
measured directly with a hydrometer (Figure 4). A
hydrometer is a bulb-type syringe which will extract electrolyte
from the cell. A glass float in the hydrometer barrel is
calibrated to read in terms of specific gravity. The lower the
float sinks in the electrolyte, the lower its specific gravity.
Fig. 4 Battery Hydrometer
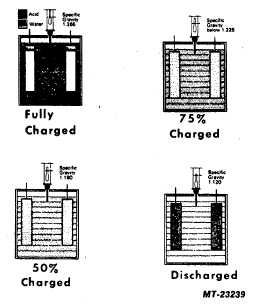
Figure 5 graphically illustrates the relationship between
specific gravity readings and the combination of the sulfate
from the acid with the positive and negative plates for various
states of charge. The black dots represent the sulfate radical.
A fully charged battery has all of the sulfate in the acid. As
the battery discharges, some of the sulfate begins to appear
on the plates. The acid becomes more dilute and its specific
gravity drops as water replaces some of the sulfuric acid. A
fully discharged battery has more sulfate in the plates than in
the electrolyte. Note that the hydrometer float sank lower and
lower in the electrolyte as the specific gravity became lower.
Table 1 illustrates typical specific gravity values for a cell in
various stages of charge. A fully charged specific gravity of
1.265 corrected to 26.7° C (80° F) is assumed.
TABLE 1
Specific
State of
Gravity
Charge
1.265
100 % charged
1.225
75 % charged
1.190
50 % charged
1.155
25 % charged
1.120
Discharged
Fig. 5 Relationship of Specific Gravity To Transfer of Sulfate
From Electrolyte To Plates.
HOW TO USE A HYDROMETER
Figure 6 illustrates the correct method of reading a
hydrometer. The barrel must be held vertically so the float is
not rubbing against the side of it. The electrolyte should be
drawn in and out of the hydrometer barrel a few times to bring
the temperature of the hydrometer float and barrel to that of
the acid in the cell. Draw an amount of acid into the barrel so
that with the bulb fully expanded, the float will be lifted free,
touching neither the side, top or bottom stopper of the barrel.
When reading the hydrometer, your eye should be on a level
with the surface of the liquid in the hydrometer barrel.
Disregard the curvature of the liquid where the surface rises
against the float stem and the barrel due to surface tension.
Keep the float clean. Make certain it is not cracked.
Never take a hydrometer reading immediately after water is
added to the cell. The water must be thoroughly mixed with
the underlying electrolyte, by charging, before hydrometer
readings are reliable. If a reading is being taken immediately
after the battery has been subjected to prolonged cranking, it
will be higher than the true value. The water formed in the
plates during the rapid discharge has not had time to mix with
the higher specific gravity acid above the plates.
CTS-2771 Page 6
PRINTED IN UNITED STATES OF AMERICA
|